EpiVax and Massachusetts General Hospital Advance on T cell-Targeted Epitope Vaccine for Q Fever
COVID Vaccines, Therapeutics, Variants, Long COVID, Ability To Tackle the Next Pandemic
A new platform for immunotherapeutic RNA delivery to cancer cells
Immune cell ‘Soldier’ identified as potential target for immunotherapy
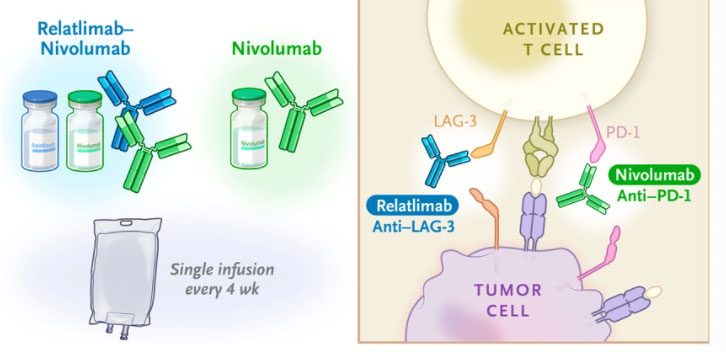
Dual-drug treatment offers promise for advanced melanoma patients
Novel drug combination may boost chemoimmunotherapy response in bladder cancer patients
A proof-of-concept study suggests that the combination of anti-inflammatory medication and chemotherapy drugs can boost immune response to suppress bladder tumor growth. Led by Cedars-Sinai Cancer investigators, the study focused on tackling the immune-dampening effect of chemotherapy drugs. Research uncovered the underlying mechanism that results in chemotherapy treatment failure, the release of prostaglandin E2. This bioactive lipid is associated with inflammation and inhibits dendritic maturation. A preceding factor for cells to fight cancer. In addition, early investigation revealed potential in combining celecoxib, an anti-inflammatory medication, and chemotherapy drugs as improved immune response was observed in treated mice models. Researchers look to test the efficacy of the potential treatment in human trials for bladder cancer patients.
Two-pronged immunotherapy treatment tested for effectiveness against glioblastomas
Recent data published indicates great hope in a newly developed COVID-19 vaccine
Developed by City of Hope researchers, an investigational COVID-19 vaccine was seen to produce both antibody and T cell response against the virus in clinical trials. The vaccine, COH04S1, differs from previous vaccines developed to target COVID-19. COH04S1 targets two different proteins of the virus, the spike and nucleocapsid proteins. This combination of antigens into one vaccine grants substantial T cell immunity. Even as new mutations arise in the virus’ spike protein, antigens of the nucleocapsid would protect individuals from COVID-19. The vaccine is currently undergoing phase 2 clinical trials, being evaluated for both efficacy for immunocompromised cancer patients and as a Phase 2 vaccine booster.