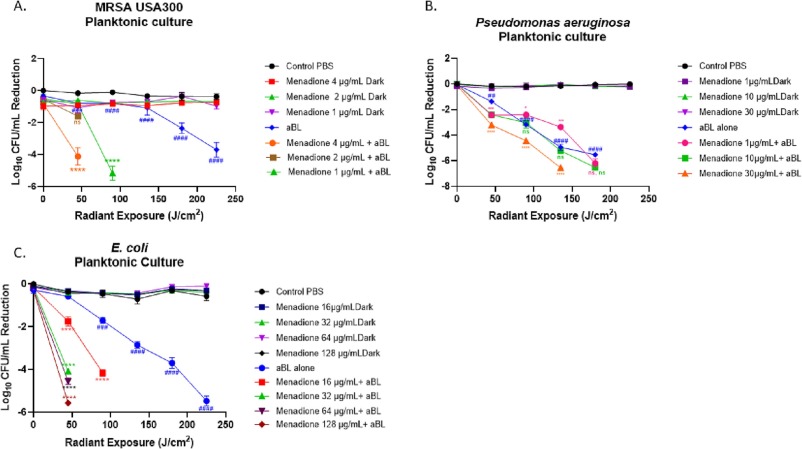
Jeffrey Gelfand M.D., principal investigator at VIC, recently published research results that show menadione (Vitamin K3) acting as a photosensitizer that increases the effectiveness of antimicrobial blue light therapy, specifically enhancing the microbicidal effectiveness of the light therapy, in treatment of biofilm infections. Findings indicate the modality as a potential alternative to antibiotic therapy, with the possibility of avoiding further development of antimicrobial resistant bacteria in biofilm infections.
We’re not ready for the next pandemic.
We cannot ignore the possibility of another pandemic, especially with the potential for more infectious and fatal disease outbreaks. Pandemic prevention is essential to protect the health and well-being of the American people. The article below from the Boston Globe mentions Dr. Mark C. Poznansky and Dr. Michael V. Callahan of the Infectious diseases department at Massachusetts General Hospital, who both urge for a definite need from national institutions to prepare an organized response for the next pandemic.
Customizing T Cell-Based Immunotherapies in a ‘SNAP’
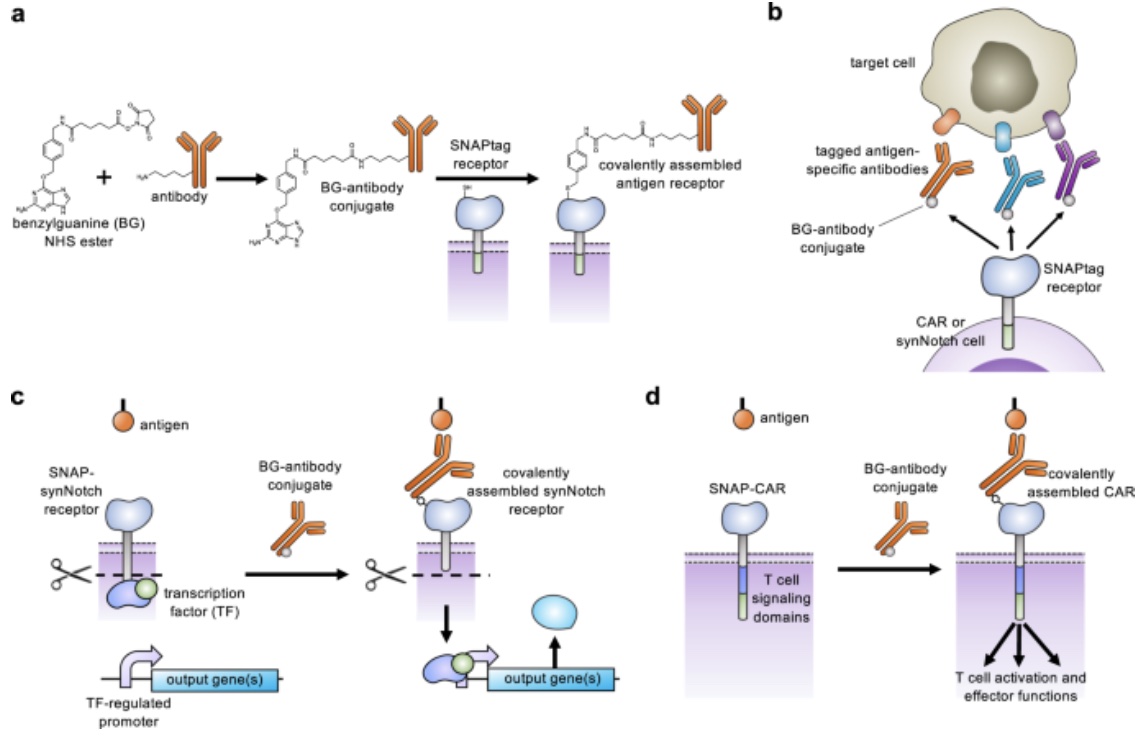
Through expanding the targeting capabilities of chimeric antigen receptors (CARs) and synthetic Notch (synNotch) receptors, University of Pittsburgh researchers have developed a “universal” receptor system that allows T cells to recognize any cell surface target. Given the potential previously seen in engineered antigen receptors, and CARs being the most clinically advanced of these technologies, the team of researchers began investigating a method to gain additional control over CAR function. By engineering T cells with receptors bearing a universal “SNAPtag”, CARs bind to the common tag molecule fused to the antigen-specific antibody, instead of directly binding antigen targets. This new approach allows for great expansion to antigen targeting and shows great promise in using CARs against additional immune-related diseases and other types of cancer.
New Research Shows That Bacteria Get “Hangry,” Too
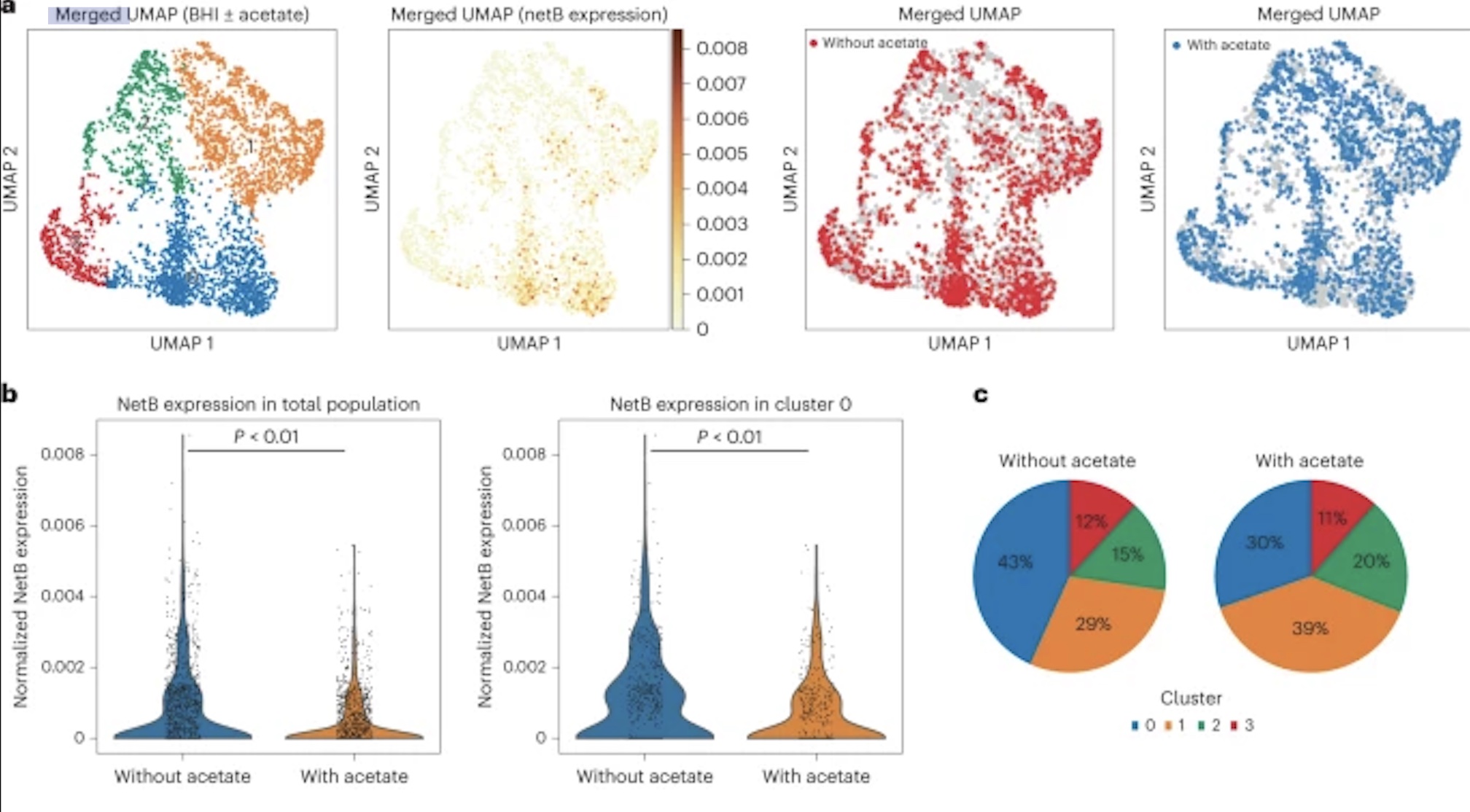
New research by Adam Rosenthal, PhD, assistant professor in the Department of Microbiology and Immunology at UNC, analyzed the cell states of isogenic bacterial populations via a developed probe-based bacterial sequencing (ProBac-seq) method. After confirming the sequencing method accurately identified known cell states and transcriptional heterogeneity of bacterial cells, Rosenthal et al. applied ProBac-seq to Clostridium perfringens and found that despite transcriptional heterogeneity, these identical cells behaved differently with varied functions within the bacterial population. Specifically, through single-cell analysis on C.perfringens and graph-based clustering, four distinct subpopulations were revealed, with all clusters preferentially expressed netB, a toxin responsible for necrotic enteritis disease. To investigate mechanisms affecting expression rates of the toxin, C.perfringen was treated with sodium acetate. Following treatment, the expression of netB was significantly less in comparison to the control cluster. These findings are just the beginning to understanding bacterial populations and mechanisms behind single-cell behavior and could lead to a new approach to antibiotic tolerance down the line.